Neuralink’s Bold Advancement This 2024
The concept of implanting chips in the human brain has transitioned from sci-fi movies and books to real life. This is due to ground-breaking efforts of researchers and tech companies that hope to revolutionize treatments for everything from cognitive disabilities to neurological issues. However, celebrating this advancement’s possible applications and benefits also involves ethical considerations.
Recently, Elon Musk posted on X that his neurotechnology company Neuralink has implanted its first human brain chip. The tech mogul added that the procedure showed promising results, including detecting “neuron spikes”, which indicates brain cell activity.
This is a huge step, especially for Neuralink, which Musk founded with other neuroscientists eight years ago, to create a device that can be implanted in the brain. This chip, which the company hopes will eventually be commercially available, facilitates communication between the brain and a computer. It’s the flagship project of Neuralink, which Musk describes as a company that enhances human biology by integrating it with computers and artificial intelligence (AI).
Meet Telepathy
The brain-computer interface is dubbed “Telepathy” because it allows people to control a computer and phone solely by thought. How does this work? Implanting requires a robot to surgically put the chip in that part of the brain that controls one’s will to perform physical action.
The chip-equipped Neuralink device utilizes a lithium battery that supports wireless charging. It contains electrode networks composed of over a thousand ultra-thin and pliable conductors, which a surgical robot incorporates into the cerebral cortex. These electrodes are engineered to detect motion-related thoughts, enabling them to command a computer or mobile device by thinking. Musk envisions this technology to empower and aid individuals with quadriplegia—a paralysis that affects the limbs and is typically caused by a spinal cord injury in the neck region.
Meanwhile, the surgical robot resembles a sewing machine equipped with sensors, cameras, and a needle finer than a single strand of human hair. According to the Neuralink website, the implant’s threads are so delicate that humans cannot insert them. Using robotics ensures accuracy and reliability in completing a highly intricate and sensitive task.
In May 2023, Neuralink secured clearance from the Food and Drug Administration (FDA) to conduct product testing via human clinical trials. The company began employing patients suffering from quadriplegia, particularly those affected by amyotrophic lateral sclerosis (ALS) and cervical spine trauma.
Before the FDA approval, Neuralink publicly demonstrated the device through a macaque in 2021. The macaque is a type of monkey similar to humans in terms of anatomy and physiology. Like humans, macaques are highly sociable and adaptable.
According to the company, the animal could direct a computer cursor toward its target through brainwave activity. Once the macaque hit the goal, it was rewarded with a dollop of banana puree. The company also showed the animal playing an arcade-inspired game using only its thoughts.
According to Musk, the clinical trials involving humans will take around six years to finish. Neuralink has yet to disclose how many patients were approved by the FDA. However, the company disclosed that with its first human trial, it hopes to assess the device and surgical robot’s safety while evaluating the technology’s ability to interpret brain activity. The regulation board would also need to review the software.
Earlier efforts on brain implants
Brain implant research and studies on similar technologies have been in progress for many years. Neural implants have met success among patients who frequently experience seizures unmanageable by medications.
Another company working on synergizing the brain and computer is Synchron, which, like Neuralink, aspires to aid those struck with severe paralysis. Since it received the FDA’s go-signal for human trials in 2021, Synchron has tested its invention on patients who sent text messages through intention, eliminating the need for typing. Meanwhile, the Pentagon’s agency, Darpa, is testing Silent Talk, a program that lets users correspond in the combat zone using brain signals instead of verbal communication.
What makes Musk’s initiative different? Financial resources can make all the difference, especially when time is of the essence. There has already been extensive research on how the motor cortex functions, enabling today’s experimentation on brain-machine interfaces to have a solid knowledge base. With study and money combined, Neuralink increases its chances of developing a commercially viable product.
Controversies surrounding Telepathy
Though Telepathy looks promising, it has its fair share of controversies.
One of these is Neuralink’s alleged mistreatment of animals and possible exposure of humans to disease-causing microorganisms via infected hardware. It was said that internal personnel also reported the hurried testing of animals, which led to their unnecessary suffering and deaths. According to Reuters, this resulted in a Department of Agriculture probe. The news article also mentioned that since 2018, Neuralink has terminated at least 1,500 animals, including monkeys, sheep, and pigs. Meanwhile, in its blog, Nueralink emphasizes its humane and ethical treatment of study animals.
Aside from this issue, the federal scrutiny also included the company’s transportation of toxic substances.
Though Musk remains optimistic about results, experts quickly warn about his definition of success. Data must be fully revealed with in-depth studies to gauge the project’s effectiveness and viability. Brain stimulation is a complex process that needs to be done in a precise manner.
Additionally, even if the device is approved for human safety, experts say it may take years before it’s cleared for commercial use.
Hope for Telepathy
Still, Neuralink has executed novel ideas, such as utilizing Bluetooth, which operates on a reduced bandwidth. While this cuts down information noise, it provides just enough information to regulate and control the device.
With Neuralink’s future success, treating neurological diseases may become possible. And though the technology is still on shaky ground, being able to test it on humans is a significant development. In the meantime, the FDA is keeping a vigilant eye on the tech company to ensure the safety of its human subjects. 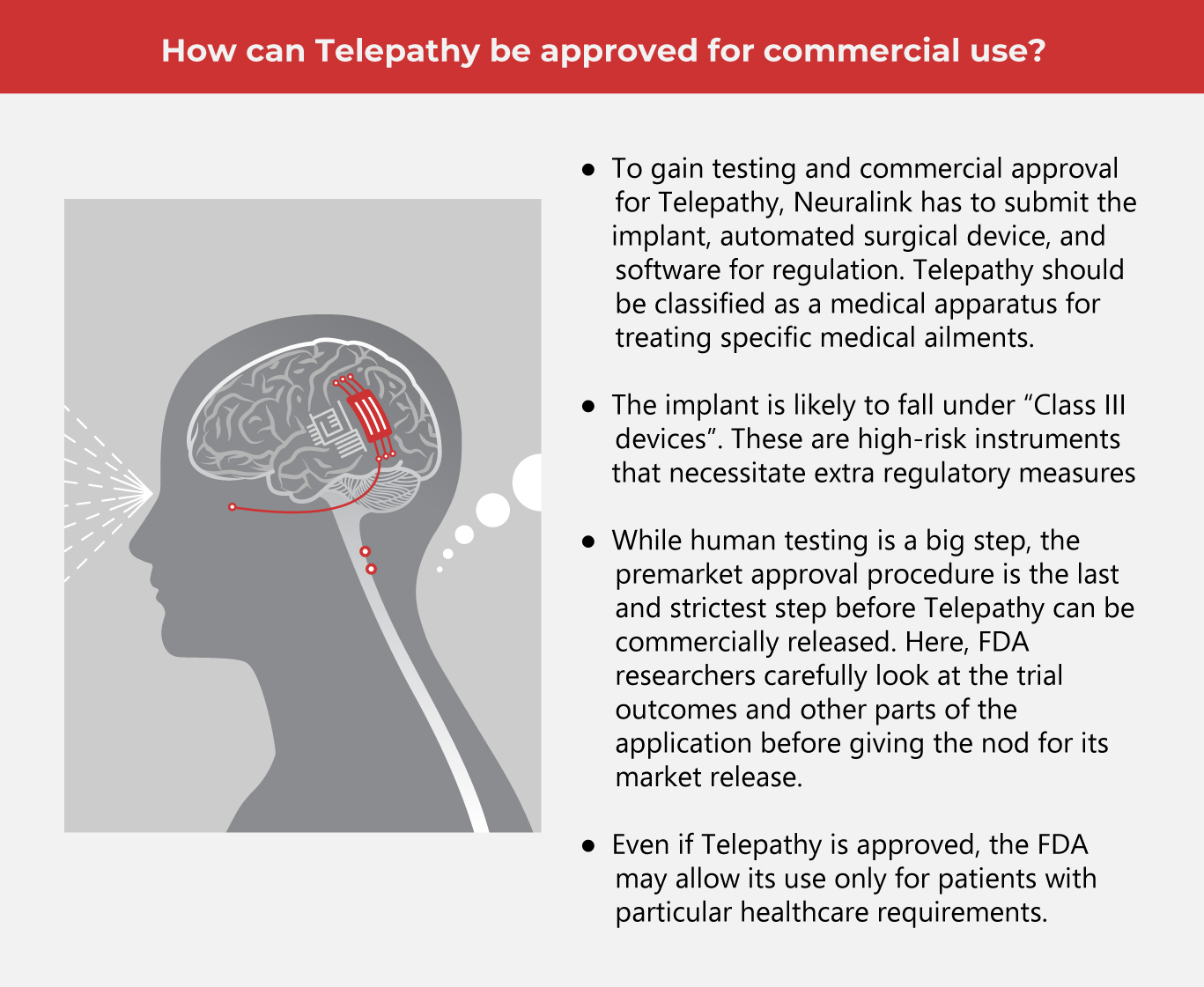
 As one of the Top 20 EMS companies in the world, IMI has over 40 years of experience in providing electronics manufacturing and technology solutions.
As one of the Top 20 EMS companies in the world, IMI has over 40 years of experience in providing electronics manufacturing and technology solutions.
We are ready to support your business on a global scale.
Our proven technical expertise, worldwide reach, and vast experience in high-growth and emerging markets make us the ideal global manufacturing solutions partner.
Let's work together to build our future today.
Other Blog